Theoretical Framework for Predicting Inorganic Fouling in Membrane Distillation and Experimental Validation with Calcium Sulfate
Abstract
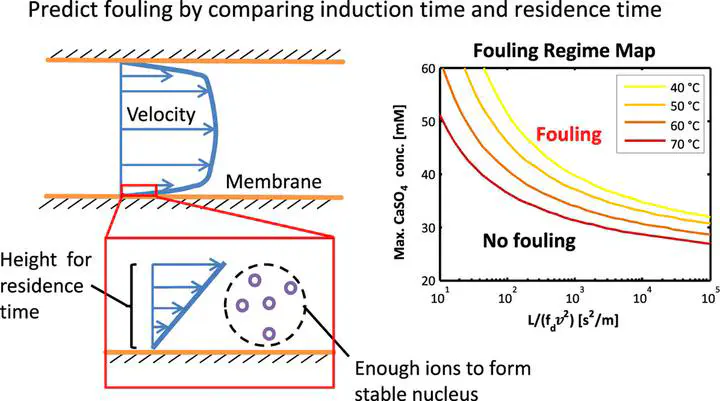
A methodology for predicting scaling in membrane distillation (MD), which considers thermodynamics, kinetics, and fluid mechanics, is developed and experimentally validated with calcium sulfate. The theory predicts the incidence of scaling as a function of temperature, concentration, and flow conditions by comparing the nucleation induction time to the residence time and applying an experimental correction factor. The relevant residence time is identified by considering a volume of solution near the membrane surface that contains enough ions to form a nucleus of critical size. The theory is validated with fouling experiments using calcium sulfate as a model scalant over a range of temperatures (40–70 °C), saturation indices, and flow rates. Although the model is validated with a bench-scale MD system, it is hoped to be compatible with large-scale systems that may have significant changes in concentration, temperature, and flow rate along the flow direction. At lower temperatures, the saturation index can be as high as 0.4–0.5 without scaling, but the safe concentration limit decreases with increasing temperature. Increasing the feed flow rate reduces concentration polarization and fluid residence time, both of which decrease the likelihood of fouling. The model is translated into easily readable maps outlining safe operating regimes for MD. The theory and maps can be used to choose safe operating conditions in MD over a wide range of conditions and system geometries.